I am Dr. Diana Zuckerman, president of the National Center for Health Research. I am speaking on behalf of our nonprofit Research Center and on behalf of members of the Patient, Consumer, and Public Health Coalition, which includes nonprofit organizations such as the National Consumer League, the Union of Concerned Scientists, and Jacob’s Institute of Women’s Health. Our Center does not accept funding from pharmaceutical or medical device companies, so I have no conflicts of interest.
Our Center became interested in Essure when we heard from numerous women who reported serious health problems from the device. We then looked at the data about Essure on the FDA website and were surprised to see that the device was described as 99.8% effective and with very few side effects. The data did not seem to reflect what the women were saying, so we were especially concerned when several of the women told us that they had participated in the clinical trials that FDA had used as the basis of their approval decision. The women told us that their reports of unrelenting, debilitating pain and other serious side effects were not included in the clinical trials.
We were even more concerned to read a study by Yale medical school faculty members indicating a 10% pregnancy rate for Essure – much higher than that for tubal ligation and long-term contraceptives.
We decided to conduct our own study, focused on the types of problems the Essure patients were describing. We included over 1,100 women who had problems with Essure and completed our questionnaire. This is not a random sample, but it gives a good indication of the patterns of health problems the Essure women are reporting.
Our Study of 1,104 women with Essure Problems
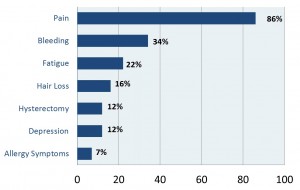
Note: please be sure to click images in order to see them more clearly.
Approximately one-third of the women in our study had their Essure devices removed, and half of those had a complete recovery. Only about 5% did not recover at all, which suggests that for almost all of the women, the Essure was the cause of their pain and other problems.This table only includes the most frequently reported problems. 86% of the women reported serious pain and 34% reported bleeding problems, which usually involved heavy bleeding. Some women told us that they were bleeding every day of the month, month after month. Many of the other frequent symptoms were autoimmune reactions: fatigue (22%), hair loss (16%), and even depression (12%) can be caused by an autoimmune reaction. Twelve percent reported having hysterectomies – remember these are very young women, usually in their 20s or 30s. Seven percent reported allergy symptoms, either from the nickel or other materials in Essure.
How could this small device cause so much damage? Here is a photo of one of the women’s Essure implants after they were removed from her two fallopian tubes. As you can see, they are stretched out of shape and broken in several pieces, some of which are still in her body.

The results of our study are quite consistent with the adverse reaction reports that the FDA has presented today, but they seem quite different from the data FDA reported. So we took another look at some of the tables that the FDA provided to you for today’s meeting.
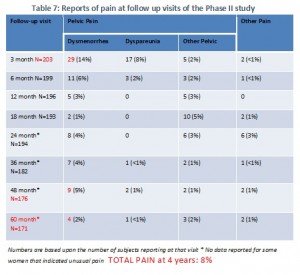
This is Table 7 from the FDA summary, but we simplified it and highlighted some of the findings in red, and also added some of the findings at the bottom. As you can see, quite a few of the women were lost to follow-up. The women told us that when they had terrible problems with pain and told their doctors about it, the doctors told them that the pain was unrelated to Essure and that they should seek medical care elsewhere. They were then often dropped from the study. If you look at the number on this table, you can see that while the number of women reporting pain seems to decrease over time, that could be explained by the substantial number of women who are no longer in the study: which started with 203 women and ended with 171. The decrease in the number of women is very similar to the decrease in the number of women reporting pain in this table. There is no way to know if they are the same women, but based on what women in the trial have told us, at least some of them were dropped from the study after they reported having chronic pain.
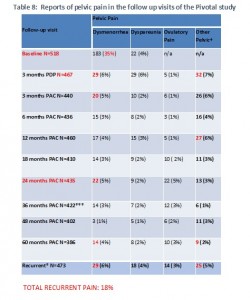
Our simplified version of Table 8 from the FDA summary shows a similar pattern. The number of women reporting pain is high 3 months after insertion and still high at 2 years, but decreases after that, at the same time that the number of women in the study is dropping dramatically. Even so, the number of women reporting recurrent pain is high. Again, if one adds all the different types of pain reported, it is very high – 18%. But, the FDA and perhaps the company have not provided the kind of information we need in order to know if we can add all the different types of recurrent pain or if there is overlap.
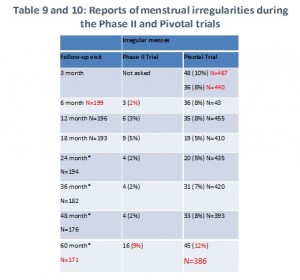
We combined Tables 9 and 10 from the FDA summary document to create one simpler table. As you can see, irregular menstruation was a problem for women in both these studies. The women tell us that usually meant very heavy, sometimes painful menstruation. In some cases, it continued all month long or most of the month.

Here you can see where one of the women in the clinical trials, Kim Hudak, reported her serious health problems, which started soon after getting Essure, and they are written down on her questionnaire. Then they are crossed out, changed to make it seem that Kim was very satisfied with Essure, and initialed by the nurse gathering the data. The initial and date means that it is an official change. When Kim later found out about this and asked why the changes were made, she was told it was because her pain and complications were unrelated to the device. But the entire purpose of clinical trials is to gather information about possible side effects, even unexpected ones. They should have been included in the study, in order to accurately evaluate how many women experienced these complications. Obviously, the change in her data to show she had an excellent experience with Essure does not reflect the years of pain and problems that Kim reported as part of the study, and to you in her statement today.
In conclusion, there are several issues of importance that face you today. How safe and effective is Essure, and compared to what? How is it that Essure was not compared to tubal ligation or long-term contraceptives but instead was studied without any comparison group?
But even if it had been compared to other treatments, the accuracy of the data is a very important issue. The data indicate that Essure is a very safe and effective product, but the women tell an entirely different story. The women know their own bodies and they know that the pain and other complications they experienced after Essure were new and different. Moreover, some of the women in the clinical trial tell us that their data were changed or that they were thrown out of the study when they reported serious problems. That is consistent with what the patients and doctors told us that doctors told their Essure patients: that their pain and complications were unrelated to the device. That consistency between what the patients were told (pain and complications was unrelated), what the patients in the clinical trials say (their reports of complications were not recorded and they were dropped from the study), and what the data say (the women were satisfied and few women reported any side effects) support the likelihood that the data were compromised. The only question is how often did that happen? Should we believe the data, or should we believe the women who were in the studies?
Another key issue is that the company is selling an implanted device, with FDA approval, without having a protocol in place for removing it safely. Any implanted device can cause problems, and it is absolutely crucial that the company and the FDA determine how best to remove it safely if it needs to be removed. Neither the company nor FDA have done so. In fact, doctors who inserted Essure in women in the clinical trials did not know how to safely remove the device when they were asked to do so. This lack of a safe protocol greatly exacerbated the problems the women had with Essure. In some cases, the failure to acknowledge that Essure was causing the problems, as well as the lack of information about how to remove it safely, turned short-term debilitating pain into debilitating pain that lasted for many years and ruined women’s lives. You’ve heard compelling examples of that today.
The final question is: if the product is to be on the market, who should do the studies to accurately determine the short-term and long-term effectiveness and side effects? The company’s track record and response to the women’s reported complications is not acceptable. Any additional research should be conducted independently of the company so that patients can have confidence in its accuracy.



